
Let’s start with the SWITCH 1 trial. What is it? It was a Phase 3a trial to compare the hypoglycaemia benefit of the two insulins. It was a double-blind, crossover trial, which essentially means that when participating, you used both insulins and didn’t know which one you had at any point in the trial. The results were published much earlier this year, but the interesting point in the study seems not to have been picked up on by many in the diabetes world.
If you want the full details of the trial design, please take a look here (you will need a login until the 17th September, after which access will be free). It’s important to note that for this trial, there were 501 initial participants and that 388 of them had factors that increased their risk of hypoglycaemia (including hypo unawareness, the experience of more than one severe event in the preceding 12 months, moderate renal failure, diabetes for longer than 15 years and a hypo in the previous 12 weeks – so what might be considered a normal Type 1!). There was a 16-week titration period followed by a 16-week maintenance period, where the basal level was considered to be correct. At the end of each 32 week period, the treatments were swapped so that all participants used both treatments.
If you want to see the results, please click here. I think you’ll be unpleasantly surprised. I was.
What was being compared?
The hypoglycaemic effects of Degludec (Tresiba) versus those of Glargine (Lantus; Basaglar) were being compared, and one might argue that the results are disturbing. Why? Because there is a clear difference in what was determined much to the detriment of Glargine. And one that should make decisions on choice of insulin very clear.
Overall Hypoglycaemic Risk
What you can see here is that during the maintenance period, once the insulin was properly titrated, there was an 11% lower rate of hypos with glucose levels below 3.1mmol/l (which I’d consider way too low!) with Tresiba. That’s a relative value and looking at the absolute numbers, shows up as roughly 7 events per patient on Tresiba and 8 events per patient on Lantus/Basaglar. Just to be clear, these are events where the participant is aware of the hypo, not ones where the participant was unaware.

But what happens when we take a look at Nocturnal and Severe hypos?
Nocturnal Hypo Risk
Well, for Nocturnal hypos there’s a 36% reduction in hypo rate with a properly titrated dose of Tresiba versus Lantus!

That’s a huge difference. It’s worth noting that Nocturnal Hypo events covers both the <3.1mmol/l events and severe events, and that whilst as a relative number, it’s enormous, the absolute numbers show up as fewer than 1 nocturnal hypo per patient on Tresiba over the maintenance period, versus around 1.3 per patient on Lantus/Basaglar.
What this means? If you suffer from nocturnal hypos a lot, and you’re using a Glargine based insulin which is properly basal tested, it may be worth asking for a change.
Severe Hypo Risk
And Severe Hypos? The worst ones, that require intervention from another person or result in an ambulance visit, or in the worst case a trip to hospital? It’s almost as bad a story as the nocturnal hypos. There was a 35% lower rate of Severe hypos in the properly titrated Tresiba than there was in the Lantus cases. Let’s remember that both insulins were used with all participants, so this isn’t participant group specific. Want to see the evidence?
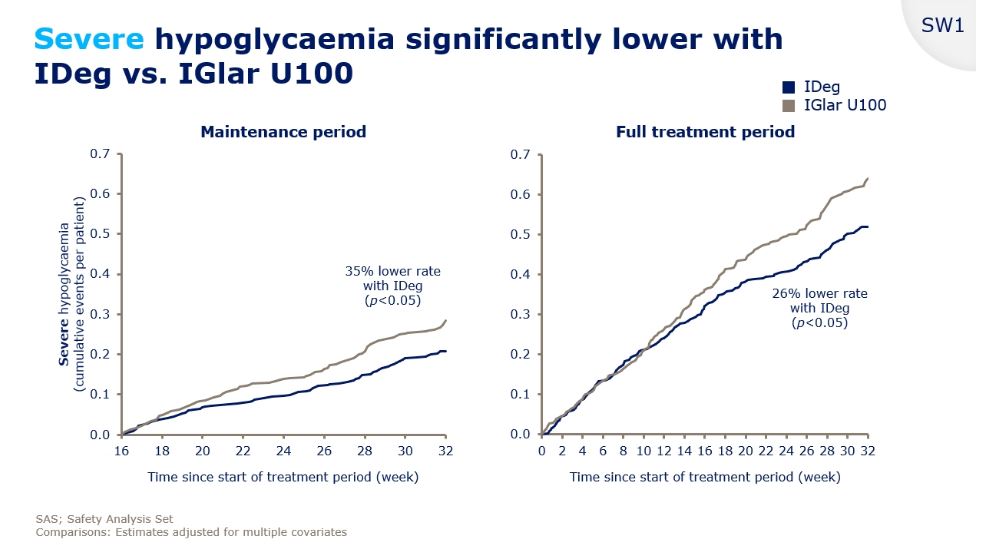
You are reading that correctly – amongst the 404 participants that completed both , there was a 35% lower incidence of severe hypos with Tresiba. Or a more than 50% greater rate of severe hypos when using Lantus compared to Tresiba.
Of course, those numbers are relative, so taking it at an absolute level, that was slightly less than 0.2 events in 16 weeks for the Tresiba treatment per user and slightly less than 0.3 events in the sixteen weeks per Lantus/Basaglar user.
What’s the relevance of this? Sixteen weeks is around 1/3rd of a year. Linearly extrapolating those numbers suggests that a Lantus/Basaglar user could expect to have a severe hypo roughly every year. A Tresiba user would expect this to happen roughly once every eighteen months.
Conclusions

The slide says it all. Lantus/Basaglar had a much higher rate of nocturnal and severe hypos in this study. A greater number of severe hypos means a much greater cost to the healthcare services of dealing with them. And in the UK, let’s remember from this investigation, that means:
- The cost of an ambulance call out is roughly £240
- Cost of a bed day (2013/2014 data) is roughly £700
- Cost of an A&E visit (2013/2014 data) is roughly £114
Then let’s consider the cost of the insulins. Tresiba is more expensive. Yes, but is it the cost of a few ambulances call out more expensive? The cost of QOL and Hypo-fear more expensive? The cost of someone living in such fear they’ll go low that they run high enough to risk complications more expensive? No. It isn’t.
And lastly, there’s the little issue of severe hypos and holding a UK driving license. If you have two hypos requiring assistance in a 12 month period, you’re supposed to tell the DVLA and they’ll rescind your license. By prescribing an insulin that increases the likelihood of this, is the NHS opening itself up to legal action?
Given the anecdotal evidence relating to Lantus/Glargine and now the outcome of this study, I wonder why Glargine (whether it’s Lantus or Basaglar) continues to be prescribed. The double-blind crossover data reports a higher incidence of issues with Glargine, which when applied across a population demonstrates a higher risk with the insulin, and I think we’ve kind of known that for a while.
This is some rather uncomfortable evidence to corroborate those reports.
All the data used by the NHS and collected from patients suggests that the majority (not the Diabetes Online Community in other words) are most afraid of Hypoglycaemic events, then given the choice of two insulins, one of which statistically is more likely to cause those events than the other, which should be offered? I’d say it was no-brainer. Even at a higher price.
Imagine, if you will, your endocrinologist saying the below to you when putting you on insulin:
“We’re putting you on Basaglar as it’s the cheapest long acting insulin on the market. We know you’re 50% more likely to have a hypo requiring assistance on it than on this alternative we have, and therefore more likely to put your driving license at risk, and that this means you’ll have roughly one hypo requiring assistance every year, but that alternative? It costs too much.”
The price to the individual of the alternative mustn’t be ignored.
Leave a Reply