
If you’ve been following the ADA Scientific Sessions, you’ll have seen lots of big device announcements and AID information, but you may have missed this little gem.
Novo Nordisk announced on Monday that at the end of their phase 2 trial, the once weekly insulin, Icodec had performed AS WELL AS LANTUS in their 26 week trial with 247 people with Type 2 Diabetes.
So, what is insulin Icodec?
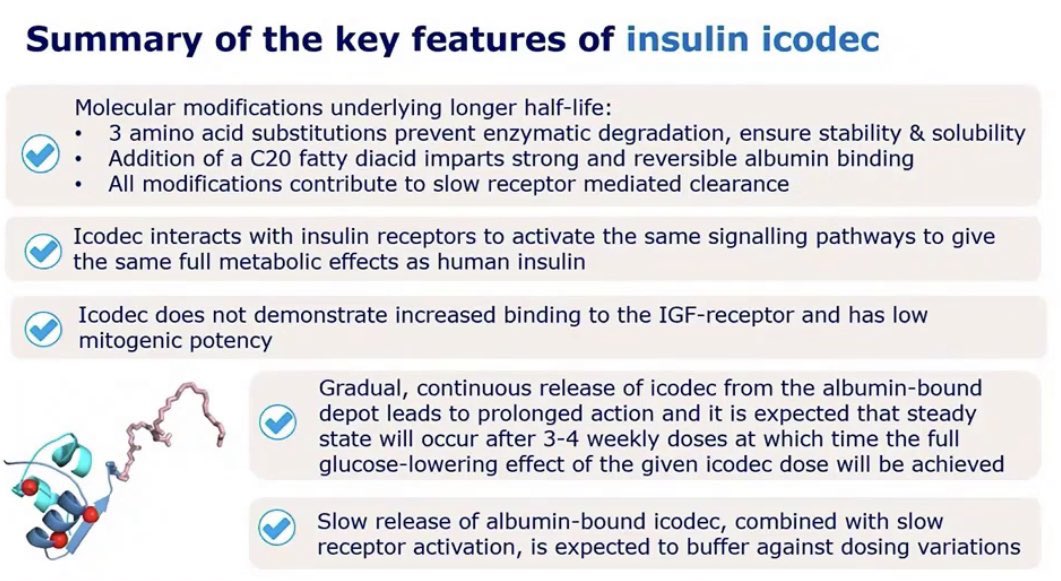
Well, it’s an investigational, long-acting basal insulin analogue with a half-life of 196 hours. Yes, that’s a half life of more than 8 days. It has also gone under the name IA287 in the past, and other trials (including one in people with Type 1) can be found with this name.
Once injected, insulin icodec binds strongly but reversibly to albumin, (a similar behaviour occurs in both Detemir and Degludec, but not to the same extent). This results in a continuous, slow and steady reduction of blood sugar over the week. The injection volume of once-weekly insulin icodec is equivalent to daily insulin glargine U100 due to the concentrated formulation that it uses.
It is intended for use amongst both type 1 and type 2 users.
And the trial?
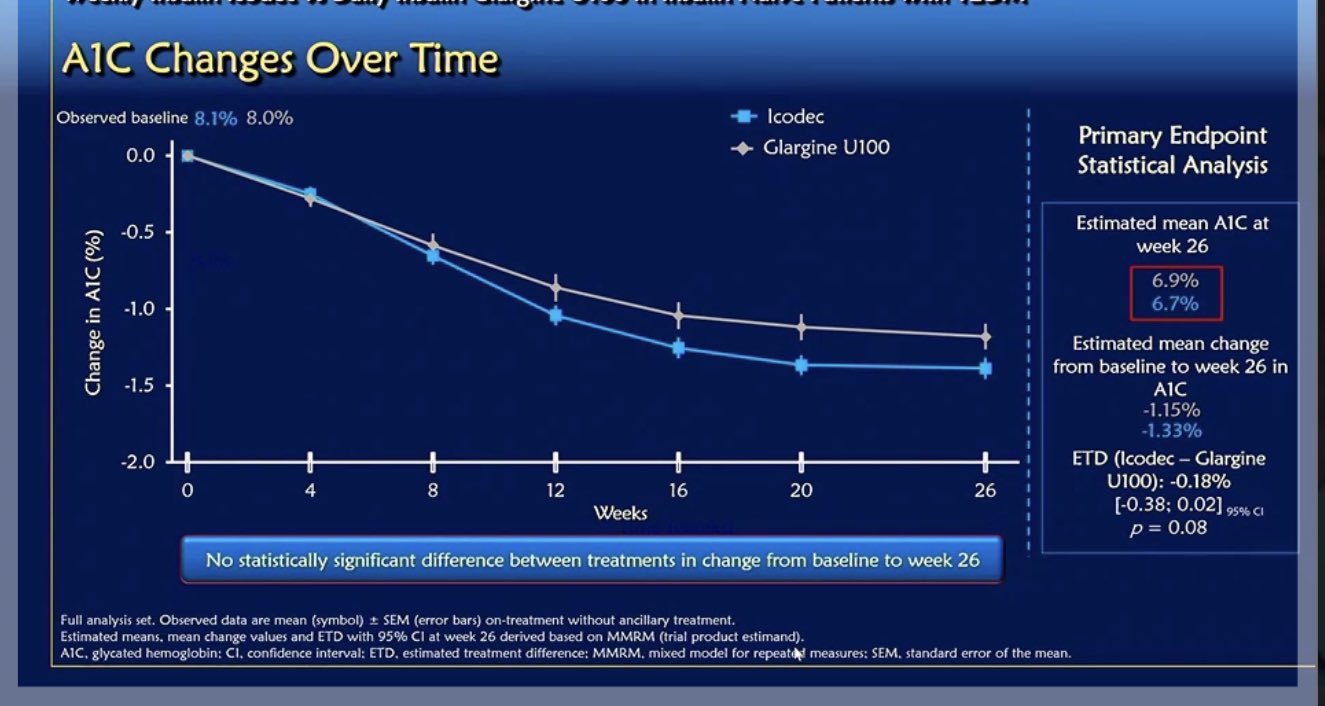
It was a randomised, double-blind, double-dummy, treat-to-target trial of once-weekly insulin icodec compared with once-daily insulin glargine U100* involving 247 insulin-naïve adults with type 2 diabetes who were unable to maintain euglycaemic levels with metformin and/or DPP-4i. The primary endpoint was change in HbA1c from baseline to Week 26. Secondary endpoints included change in fasting plasma glucose from baseline to Week 26 and hypoglycaemic episodes.
The previous trial in people with type 1 diabetes
The results?
The results were remarkably impressive. The primary endpoint showed that the change from baseline to week 26 in HbA1c was similar in participants receiving once-weekly insulin icodec compared to once-daily insulin glargine U100 (-1.33% vs -1.15% points, respectively, p=0.08). Secondary endpoints included change in fasting plasma glucose (FPG) from baseline to week 26, which was similar for insulin icodec and insulin glargine U100 (-58mg/dL and -54mg/dL respectively), and the change from baseline to week 26 of the mean of the nine-point self-monitoring of blood glucose (SMBG) profile, which was greater for icodec (-7.9 mg/dL; p= 0.01).
Overall, for those with Type 2, this once per week background insulin appears to have worked really well.
Perhaps more importantly, there appears to be an ongoing trial with people with Type 1 which is due to complete later in June. Maybe that will be something we see at EASD later this year…
However you look at it, for many people, one basal shot per week can only be a good thing and will making living with any form of diabetes a much easier experience. Roll on the Type 1 trial data…
Thank you for providing this interesting report. Whilst I can understand the advantages of a very long lasting insulin what concerns me is the adaptability of such a regime to the actual daily routine of Type one diabetics. For example how is episodic exercise managed with for some diabetics where a resultant drop in BSLs some hours after the exercise, or if insulin requirements increase during stress, illness etc where any adjustment to such a long-acting insulin might take more than a week to produce changed insulin levels. I do however like the fact that this insulin is albumin bound and thus, I assume, not likely to cause severe hypoglycaemia if accidentally injected into a blood vessel or presumably a risk with intramuscular injection also. And I wonder about what the actual dose of this insulin would be for the week?
All these reports etc r given by big pharma so the results r questionable
Sounds fantastic as a diabetes 1 on daily insulin injections
Hope it happens?
Colin Tudhope
Port Melbourne
AUSTRALIA
It is a great insulin, according to tests so far.
it is.
Good summery. Seems to be an excellent options provided no unexpected hypoglycemia or any toxicity in lever.
compliance is the key behind future insulin.
Is Icodec available at pharmacy’s now
No. It hasn’t undergone phase 3 trials yet.
I have been involved with a trial for the last 4 months. While Icodec does some impressive things, it also does some things my Dr. nor anyone else will talk about.
What does it do? Most notably, it effects my short acting Novolog. Oh, I must be imagining that, right? Ive been insulin dependent since 1995. I have some experience with Novolog and Humalog. Humalog was great, until 7/2017 when I suddenly began needing TWICE the dosage for each meal. Literally, I went from needing 10 units (for example) for a meal to needing 20. My Dr. had no answer. I switched to Novolog, which I had used previously. All was returned to normal. 10 units (for example) worked like 10 units should – in an hour or less. My BG was reduced as needed. With Icodec, my Novolog is taking 3-5 hours to effect my BG, if it effects it at all. My Dr. said I was eating too much and/or taking too little Novolog. Easy fix, I ate EXACT amounts and took EXACTLY the amount they suggested (even tho’ I felt they suggested TOO MUCH) for each meal, based on card counting. Sometimes it work, most of the time it didnt. Today, for example, my BG at 430 am was 180. I did not eat a thing and took a small bolus to drop my BG a little. 3 hours later my BG was 320. I took a much LARGER bolus to offset my BG. 2 hours later my BG is 310. I have NEVER had this sort of delay with Novolog – EVER!!!!! Icodec effects insulin’s effectiveness. But, that may mean a delay to figure that out. Big Pharma wants its billion$ NOW!!! They wont fix it and they wont wait and the FDA will allow them whatever they want. If covid has taught us anything, it has taught us that Pharma is controlling medical decisions, based on their investments. Oh, dont like that? Gonna pull this comment for all its facts and truth? Typical USA “transparency”.
Where is my comment?? Dont like the truth? Icodec is a messed up insulin. It effects the effectiveness of any other insulin a patient is using. Yet, you liars dont want people to know that. So you, with all the bias of a communist, get rid of all comments that do not put icodec in a “prefect” light. What next? Hide all the patients who have diabetes complications due to high BG due to icodec messing up their fast acting insulins?? And you took the Hippocratic oath?? Well, your “doctors” did. Quick, remove this post before anyone sees the TRUTH about icodec. Fah Q